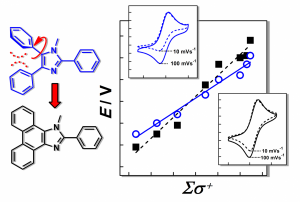
The recently published manuscript (J. Am. Chem. Soc. 2014, 136, 427–435) deals with the improvement of the properties of redox catalysts based on the triarylimidazole framework, which can be achieved by linking the ortho-carbons of the aromatics in position 4 and 5 (see figure, left). Thereby, a fused framework is generated, removing the distortion from planarity and enhancing the influence of the substituents on the redox properties. This modification leads not only to a much broader range of available redox potentials for the resulting phenanthro[9,10-d]imidazoles but also to improved stability of the corresponding radical cation. In addition, high catalytic activity was found for electro-oxidative C–H activation reactions.
September 23, 2014 - 10:22am